Keylika: Redefining the Standard of Care for Iron Deficiency
World's first prescription iron patch to treat Iron Deficiency Anemia
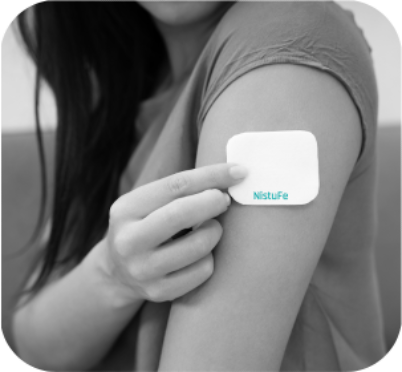
Keylika is combining its metallodrug synthesis technology with optimized delivery methods to obtain improved clinical outcomes. Our first product is a novel iron drug in a wearable skin patch with significantly improved safety, efficacy and tolerability to treat Iron Deficiency Anemia (IDA). When approved, Keylika’s proprietary transdermal iron will be the world's first prescription iron patch.
The Problem
Iron Deficiency Anemia (IDA) is a hugely understated, highly prevalent problem with subpar treatments.
Iron deficiency (ID) affects 30 million people in the US alone and about 1.6 billion worldwide. Current treatments include oral iron supplements which are typically associated with low bioavailability and poor tolerability due to mild to severe gastrointestinal (GI) side-effects (constipation, nausea, stomach discomfort, diarrhea, etc.) often leading to patient non-compliance. Also, a significant number of patients just do not absorb iron taken orally. The second line of treatment with intravenous (IV) iron infusions is more invasive, riskier, costlier and requires hospitalization due to the risk of anaphylactic shocks.
Our Solution
Our first product is a new small molecule (new molecular entity), an iron-containing drug designed for transdermal delivery by a wearable skin patch: NistuFe. It is a safe, highly bioavailable iron complex with higher efficacy. A therapeutically relevant dosage is administered through the skin directly into the systemic circulation, thereby circumventing the gastrointestinal tract and all associated adverse effects. Thus, we combine the benefits of both orals and IV iron, and without their respective downsides: a drug suitable for self-administration with no GI side effects and no required hospitalization that yields a superior treatment outcome from the convenience of a patient’s home. This is slated to be a potentially best-in-class, breakthrough therapy. Our preliminary proof-of-concept in vivo studies in rats have demonstrated excellent results.
Our Science
At our core is a platform synthesis technology we have developed for synthesizing mixed ligand metal complexes. We are using this platform to generate a range of drug candidates for treating therapeutic conditions in the fields of hematology, pain and inflammation, metabolic disorders, infectious diseases and oncology. We then administer these drugs using proven delivery technologies, to effect the best clinical outcomes. Positioned at the intersection of drug development and drug delivery, we customize the molecular structure of the drug and its formulation to optimize the therapeutic delivery per clinical requirements.
Target Population
Our first indication being menorrhagia, our primary target population is women of child-bearing age in the US who suffer from severe to moderate ID, which has an estimated prevalence of 6 million. A wide range of other patient groups adds up to a potential 30 million users in the US alone.
Our Ask
1. If you know anyone in your social circle suffering from Iron Deficiency, we would love to talk to them!
2. If you are connected to biopharma companies with interests in the hematology space, could you please introduce us?
Please follow us on LinkedIn!
Questions? Feel free to reach out: founders@keylika.com